Polycarbonate (PC) is a class of amorphous general-purpose engineering plastics, and it is also the only transparent material among all kinds of general-purpose engineering plastics. PC resin is a general term that refers to a polymer in which monomers are linked together by carbonate groups through carbonate bonds. Polycarbonate’s excellent impact resistance, transparency, heat resistance (high glass transition temperature around Tg ~ 150°C), and dimensional stability make it a good material choice for a variety of applications. On the other hand, the ester bonds in polycarbonate make it less chemically resistant than other materials (it is particularly susceptible to alkalis and aromatic hydrocarbon-based solvents such as oils), and polycarbonate is also susceptible to hydrolysis in warm, humid environments.
2. Production of polycarbonate
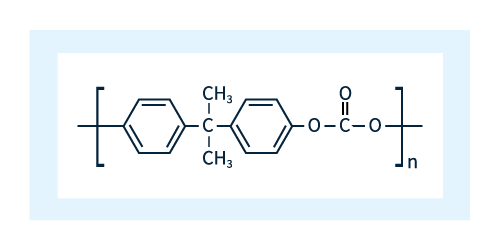
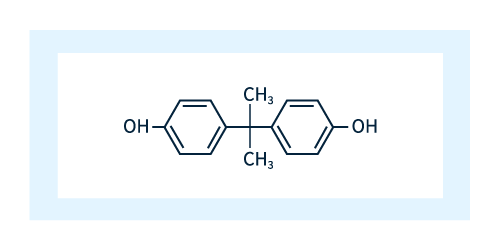
The basic chemical structure of polycarbonate is shown in Figure 1.
Figure 1: Chemical structure of polycarbonate
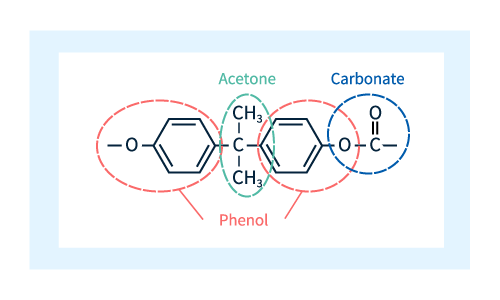
As shown in Figure 2, the unit shown in Figure 1 consists of 4 molecular components: two phenols (red dotted line), one acetone (green dashed line), and one carbonate (blue dashed line).
Figure 2: Molecular composition of polycarbonate
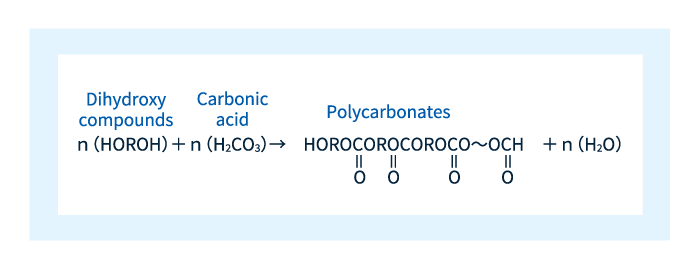
The term polycarbonate is derived from the presence of carbonate in Figure 2. More specifically, polycarbonate is a material made up of polymer chains formed by repeated reactions of dihydroxy compounds with carbonate molecules, as shown in Figure 3.
Figure 3: Reaction to the production of polycarbonate.
Changing the unit labeled R in Figure 3 can produce a variety of different polycarbonates, and the R unit used in industrial production is bisphenol A (BPA). As shown in Figure 4, BPA consists of two phenol molecules linked by acetone molecules and is a common ingredient in products such as paints and adhesives.
Figure 4: Bisphenol A
There are various techniques for manufacturing polycarbonate, which vary depending on the substance that reacts with BPA. These methods are described in the following table.
| Interface Methods (Interface Aggregation) | BPA and phosgene are mixed, reacted, and polymerized in the presence of a catalyst. This method can easily adjust the molecular weight and produce polycarbonate with excellent transparency. |
|---|---|
| Catalytic Methods (Transesterification Process) | BPA and diphenyl carbonate (DPC) are mixed, reacted, and polymerized in the presence of a catalyst. |
| Asahi Kasei’s non-phosgene process | BPA vs. the one provided by the CO 2and DPC made from ethylene oxide (EO) and polymerized. Advantages include the use of CO 2As an ingredient and does not use phosgene (a highly toxic gas) as an ingredient. Please note that Asahi Kamoto does not manufacture PCs itself, but licenses this technology to PC manufacturers around the world. |
3. Characteristics of polycarbonate
Transparency:
Of all the general-purpose engineering plastics, polycarbonate is the only transparent resin. Typical transparent material grades provide 85-90% visible light transmission (for materials with a thickness of 2 mm).
• Impact Resistance:
Of all plastics, polycarbonate has the highest level of impact resistance.
•Heat tolerance:
With a glass transition temperature of nearly 150°C, polycarbonate provides stable mechanical properties over a wide temperature range. For general non-strength reinforced grades, the typical heat deflection temperature is around 1-80°C at a heavy load of 120.130 MPa.
• Dimensional Stability:
Because polycarbonates are amorphous resins, they exhibit minimal shrinkage during molding and minimal dimensional change when absorbing water.
• Self-extinguishing characteristics:
Typical polycarbonate grades have a high flame retardancy rating of UL 94 V-2. For applications that require higher levels of flame retardancy, flame retardant additives can also be added.
• Polycarbonates are susceptible to alkalis and aromatic hydrocarbon solvents (e.g., oils).
• The ester bonds in polycarbonate make it susceptible to hydrolysis in warm, humid environments.
The most attractive features of polycarbonate are its transparency and good mechanical properties, especially its excellent impact resistance. Polycarbonate also has high dimensional accuracy, as its amorphous structure ensures minimal shrinkage during the molding process.
4. Application of polycarbonate
In terms of the amount of material used, the main applications of polycarbonate are electrical and electronic equipment, office equipment, films and sheets, and automotive parts.
In recent years, the demand for LED lighting materials for electrical/electronic equipment and office equipment has grown significantly. Polycarbonate’s excellent optical clarity and heat resistance make it an ideal material for lenses. In household and office appliances, the alloy material blended with polycarbonate and ABS is widely used as instrument chassis and housing materials. Reasons for using polycarbonate include its good moldability, ease of coloring, flame retardancy and impact resistance.
In construction and civil engineering, polycarbonate’s high clarity and excellent impact resistance make it a widely used choice for film and sheet materials. Liquid crystal display panels are another high-volume application of polycarbonate.
Perhaps the most prominent application of polycarbonate in the automotive sector is automotive headlamps (Figure 5). Modern light sources, advances in thermal design, the development of case hardening technology, and other factors have led to the use of polycarbonate in most new cars, which also contribute to overall weight reduction. Polycarbonate is also used in other transparent parts, such as instrument panels and various types of lenses, as well as exterior parts such as grilles, as well as interior and structural parts such as buttons and switches.
In addition to the applications described above, DVDs and other optical discs (Figure 5) are also made of polycarbonate; The excellent clarity and heat resistance of PC resin make it an ideal material for this application, but the growing popularity of Internet-based content distribution has led to a decline in demand.

5. Polycarbonate and modified PPE resins: comparison of main characteristics and main applications
In addition to polycarbonate, the category of amorphous engineering plastics includes:Modified PPE resin。 In this section, we will briefly compare the main characteristics of these two material families and describe the different ways in which they are used.
| polycarbonate | Improved personal protective equipment | |
|---|---|---|
| Specific gravity/weight loss | ++++ | +++++ |
| Low water absorption | ++++ | +++++ |
| Hydrolysis resistance | +++ | +++++ |
| Flame retardant | +++++ (depending on grade level) | |
| Molding properties | +++++ (depending on grade level) | |
| Electrical properties | ++++ | +++++ |
| heat tolerance | +++++ (depending on grade level) | |
| Optical properties | transparency | opaque |
| Coloring/discoloration | May be colored | Coloring may occur, but yellow discoloration may occur |
| Impact resistance | +++++ | +++ |
| Acid/alkali resistant | +++ | +++++ |
| Resistant to organic solvents | ++ | ++ |
Table: Properties of polycarbonate and modified PPE resins
Application of polycarbonate
Because polycarbonate is transparent and can be colored, it is widely used in optical components and parts that affect the appearance of products. Polycarbonate is particularly well suited for applications that require transparency and high impact resistance, and is often used for automotive components and optical components that require high heat resistance.
Application of modified PPE resins
Modified PPE resins are used in a variety of applications using their advantageous properties, including low specific gravity (which helps reduce weight), hydrolysis resistance, chemical resistance, good electrical properties, and high dimensional accuracy. Examples of such applications include peripheral components for lithium-ion battery systems, connectors for solar generators, products required for 5G communication systems, and other applications that are ubiquitous in the modern world, although they may not be obvious in everyday life.
6. Processing method
Since polycarbonate is often used in fields that require high dimensional accuracy and low shape distortion, it helps the material to flow inInjection moldingThe method of tooling and the technology to improve the surface transfer properties of the molded body are the focus of continuous development work. One example is the injection compression molding technique developed for optical discs, in which the cavity is widened during material filling, allowing the molten resin to flow more easily and then returning to its original thickness to ensure high-precision surface transfer. Another example is the development of case hardening treatments for automotive components that require high wear damage resistance.
7. Practical considerations related to the use of polycarbonate
The high melt viscosity of polycarbonate requires certain special regulations for the mold and molding process, otherwise the molded product may have defects, shape distortions, or cracks.
The chemical resistance of polycarbonate, while relatively high in transparent materials, is ultimately limited by its amorphous structure and carbonate bonds, so molding conditions need to be carefully adjusted to minimize shape distortion. Care must also be taken to avoid the adhesion of machine lubricants during the processing phase and to select a release agent that guarantees minimal residual effects. Similarly, when using molded polycarbonate products, care must be taken in the selection of sprays and cleaning products to avoid adverse side effects.
8. Polycarbonate and environmental sustainability
As in verse 2″Production of polycarbonate”As discussed, Asahi Kasei has developed a practical non-phosgene process that uses CO 2and EO as an ingredient in the manufacture of polycarbonate. The process does not use phosgene, a highly toxic gas, or methylene chloride, a suspected carcinogen, and is designed with safety in mind, and its technology has been licensed to PC manufacturers around the world.
Asahi Kasei also offers polycarbonate grades based on a mass balance approach for biomass compatibility. Although the ingredient BPA used to make polycarbonate is produced from phenol, these methods use substances derived from biomass components to produce this phenol.
The primary method of recycling polycarbonate is material recycling, in which used resin materials are ground into powder form, melted, and reshaped. This method is particularly useful for items such as second-hand discs, which tend to be of high quality and relatively easy to aggregate in large quantities.
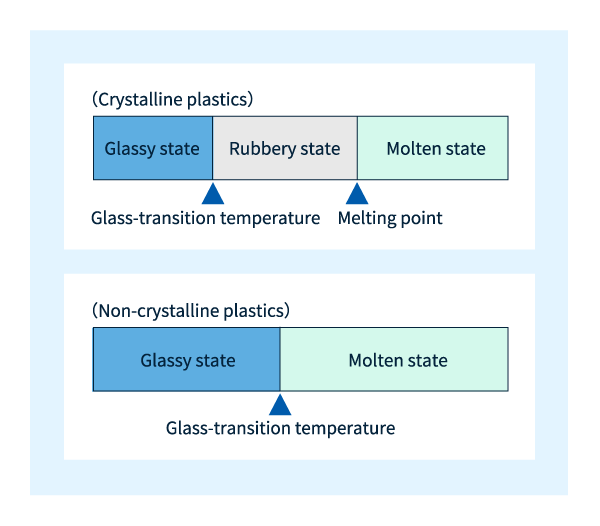
Column: Melting of crystalline and amorphous plastics
When an amorphous plastic material melts by heating it above a certain temperature, the adjacent molecules within the plastic can move freely throughout the material. The temperature at which this happens is called the glass transition temperature, which is denoted by the symbol Tg, and when the temperature is higher than Tg, the material begins to exhibit fluidity. The glass transition temperature of polycarbonate is Tg~150°C.
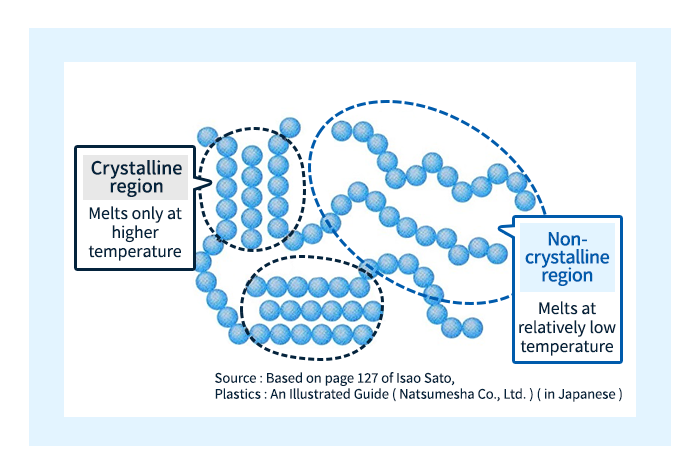
The melting of crystalline plastics is slightly more complicated. Looking at the microstructure of crystalline plastics, it can be seen that the amorphous region coexists with the crystalline region, and at higher temperatures, the molecules in the amorphous region begin to move first, while the molecules in the crystalline region are bound by strong intermolecular forces and cannot move, so they continue to exist in a solid state. As the temperature increases further, the molecules in the crystalline region also begin to move freely, and the material begins to exhibit fluidity. The temperature at which the molecules in the amorphous region begin to move freely is called the glass transition temperature (Tg) – the same term used for amorphous plastics. On the contrary, the temperature at which the molecules in the crystallization region start moving freely is known as the melting point and is denoted as Tm.
Crystalline plastics exist in a glass state at temperatures below Tg, while between Tg and Tm they exist in a rubberized state. Although glassy and rubbery plastics are both solids, there are significant differences in their properties: the molecular behavior in the former state is reminiscent of the familiar properties of glass in everyday life, while the molecular behavior in the latter state is reminiscent of one of the behaviors of rubber, thus explaining the choice term. Needless to say, for amorphous plastics, there are no analogues in the rubber state. The relationship between temperature and plastic state is shown in Figure 7.
Figure 6: Melting point and glass transition temperature